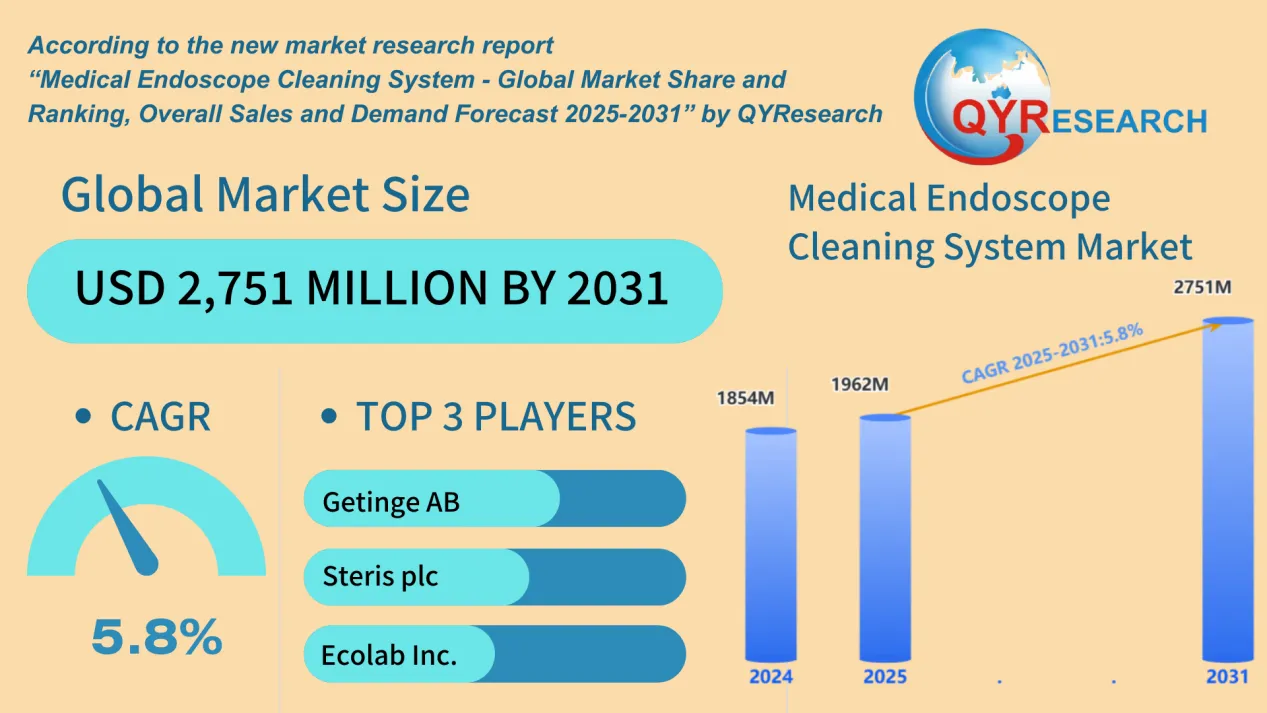
The global market for Medical Endoscope Cleaning Systems is projected to grow significantly from USD 1854 million in 2024 to USD 2751 million by 2031, according to the latest market forecast of QYResearch, with a compound annual growth rate (CAGR) of 5.8% during 2025–2031. This expansion is being driven by technological innovation, regulatory pressure for infection control, and rising global healthcare infrastructure demands.
Key Companies Leading the Market
The market is dominated by a number of key players, including:
Olympus Corporation
Getinge AB
Steris plc
Ecolab Inc.
Cantel Medical (SteriTec)
Belimed AG
Miele & Cie. KG
Steelco S.p.A.
Wassenburg Medical
Shinva Medical Instrument Co. Ltd.
These companies are investing heavily in automation, high-level disinfection, and digital traceability solutions to meet the increasing demand for safe and efficient reprocessing of endoscopes.
Recent Corporate Developments
In March 2025, Australian medtech company Nanosonics received De Novo FDA clearance for its CORIS system—an automated channel-cleaning technology for flexible endoscopes. This marks a significant step forward in infection prevention.
Meanwhile, Olympus and Canon Medical Systems have continued their collaboration into 2025 to improve endoscopic ultrasound imaging and cleaning systems, ensuring greater synergy between diagnostics and reprocessing.
Market Segmentation
By Type
Automatic Endoscope Reprocessors
Manual Endoscope Cleaning Equipment
High-Level Disinfection Workstations
Drying and Storage Systems
Others
By Application
Gastroenterology Departments in Hospitals
Operating Rooms
Endoscopy Centers
Third-Party Disinfection Service Providers
Others
Regional Insights
While North America continues to be a mature market, the Asia-Pacific region is expected to show the fastest growth due to investments in hospital infrastructure and rising procedural volumes. Europe remains strong in automated cleaning system deployment, supported by companies like Getinge and Steelco.
Emerging Trends
Automation and AI in Medical Device Reprocessing
In 2025, the medical device reprocessing industry is experiencing a technological evolution driven by the widespread adoption of automation and artificial intelligence (AI). Automated Endoscope Reprocessors (AERs) are now equipped with smart monitoring, predictive maintenance capabilities, and traceability systems, enhancing operational efficiency and regulatory compliance.
Predictive Maintenance and Smart Monitoring
Predictive maintenance solutions powered by AI are enabling hospitals and healthcare facilities to anticipate failures before they occur, reducing downtime and extending equipment life. Siemens, for instance, has integrated generative AI into its Senseye Predictive Maintenance solution to enhance diagnostics and user interaction. Real-time monitoring systems, equipped with sensors and machine learning algorithms, now continuously assess sterilization efficacy and performance, ensuring devices remain within required thresholds.
Enhanced Traceability and Compliance
To meet stringent hygiene and safety protocols, companies are implementing AI-driven data logging and visualization dashboards that provide complete traceability for every endoscope cycle. This capability not only satisfies FDA and EU MDR requirements but also supports internal audits and performance analytics, promoting safer patient outcomes.
Impact of U.S. Tariffs on Global Supply Chains
In 2025, U.S. tariff policy changes have significantly influenced global medical device trade. A universal 10% tariff on imports, coupled with elevated rates for regions like the EU (20%) and China (54%), has caused disruptions in cross-border manufacturing and logistics.
Tariff Implementation and Industry Response
These tariffs have escalated the cost of production and prompted manufacturers to reconsider offshore operations. Several leading firms are exploring reshoring strategies, developing regional supplier networks, and investing in automation to offset rising costs. For example, U.S. and European companies are increasingly localizing sterilization equipment production to avoid import duties and maintain cost competitiveness.
Strategic Reassessments and Opportunities
Despite the challenges, the situation has presented new opportunities. Manufacturers are using this disruption to digitize their supply chains, implement AI for demand forecasting, and enhance resilience through multi-sourcing strategies. According to recent industry insights, companies prioritizing digital transformation and regional flexibility are expected to outperform in the post-tariff environment.
With infection control at the heart of hospital safety, the Medical Endoscope Cleaning System market is set to expand with innovative, automated, and AI-enhanced technologies. As companies adapt to policy changes and regional dynamics, their investments in R&D and product development will shape the future of endoscope hygiene globally.
Get free Sample report:
Medical Endoscope Cleaning System – Global Market Share and Ranking, Overall Sales and Demand Forecast 2025-2031
https://www.qyresearch.com/reports/4736387/medical-endoscope-cleaning-system
Global Medical Endoscope Cleaning System Market Research Report 2025
https://www.qyresearch.com/reports/4736384/medical-endoscope-cleaning-system
Global Medical Endoscope Cleaning System Sales Market Report, Competitive Analysis and Regional Opportunities 2025-2031
https://www.qyresearch.com/reports/4736383/medical-endoscope-cleaning-system
Global Medical Endoscope Cleaning System Market Insights, Forecast to 2031
https://www.qyresearch.com/reports/4736382/medical-endoscope-cleaning-system
About Us
QYResearch founded in California, USA in 2007. It is a leading global market research and consulting company. With over 18 years’ experience and professional research team in various cities over the world QY Research focuses on management consulting, database and seminar services, IPO consulting, industry chain research and customized research to help our clients in providing non-linear revenue model and make them successful. We are globally recognized for our expansive portfolio of services, good corporate citizenship, and our strong commitment to sustainability. Up to now, we have cooperated with more than 66,000 clients across five continents. Let’s work closely with you and build a bold and better future.
Contact Us:
If you have any queries regarding this report or if you would like further information, please contact us:
QY Research Inc.
Add: 17890 Castleton Street Suite 369 City of Industry CA 91748 United States
E-mail: global@qyresearch.com
Tel: 001-626-842-1666(US) 0086-133 1872 9947(CN)
EN: https://www.qyresearch.com
JP: https://www.qyresearch.co.jp
